 |
|
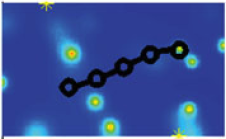
Abstract: The role of fluid motion in delivery of nutrients to phytoplankton cells is a fundamental question in biological and chemical oceanography. In the study of mass transfer to phytoplankton, diatoms are of particular interest. They are nonmotile, are often the most abundant components in aggregates and often form chains, so they are the ones expected to benefit most from enhancement of nutrient flux due to dissipating turbulence. Experimental data to test the contribution of advection to nutrient acquisition by phytoplankton are scarce, mainly because of the inability to visualize, record and thus imitate fluid motions in the vicinities of cells in natural flows. Laboratory experiments have most often used steady Couette flows to simulate the effects of turbulence on plankton. However, steady flow, producing spatially uniform shear, fails to capture the diffusion of momentum and vorticity, the essence of turbulence. Thus, numerical modelling plays an important role in the study of effects of fluid motion on diffusive and advective nutrient fluxes. In this paper we use the immersed boundary method to model the interaction of rigid and flexible diatom chains with the surrounding fluid and nutrients. We examine this interaction in two nutrient regimes, a uniform background concentration of nutrients, such as might be typical of an early spring bloom, and a contrasting scenario in which nutrients are supplied as small, randomly distributed pulses, as is more likely for oligotrophic seas and summer conditions in coastal and boreal seas. We also vary the length and flexibility of chains, as whether chains are straight or bent, rigid or flexible will affect their behaviour in the flow and hence their nutrient fluxes. The results of numerical experiments suggest that stiff chains consume more nutrients than solitary cells. Stiff chains also experience larger nutrient fluxes compared to flexible chains, and the nutrient uptake per cell increases with increasing stiffness of the chain, suggesting a major advantage of silica frustules in diatoms. |
|
Abstract: The goal of this paper is to examine the evaluation of interfacial stresses using a standard, finite difference based, immersed boundary method (IMBM). This calculation is not trivial for two fundamental reasons. First, the immersed boundary is represented by a localized boundary force which is distributed to the underlying fluid grid by a discretized delta function. Second, this discretized delta function is used to impose a spatially averaged no-slip condition at the immersed boundary. These approximations can cause errors in interpolating stresses near the immersed boundary. To identify suitable methods for evaluating stresses, we investigate three model flow problems at very low Reynolds numbers. We compare the results of the immersed boundary calculations to those achieved by the boundary element method (BEM). The stress on an immersed boundary may be calculated either by direct evaluation of the fluid stress (FS) tensor or, for the stress jump, by direct evaluation of the locally distributed boundary force (wall stress or WS). Our first model problem is Poiseuille channel flow. Using an analytical solution of the immersed boundary formulation in this simple case, we demonstrate that FS calculations should be evaluated at a distance of approximately one grid spacing inward from the immersed boundary. For a curved immersed boundary we present a procedure for selecting representative interfacial fluid stresses using the concepts from the Poiseuille flow test problem. For the final two model problems, steady state flow over a bump in a channel and unsteady peristaltic pumping, we present an 'exclusion filtering' technique for accurately measuring stresses. Using this technique, these studies show that the immersed boundary method can provide reliable approximations to interfacial stresses. |
|
Abstract: Peristaltic pumping by wavelike contractions is a fundamental biomechanical mechanism for fluid and material transport and is used in the esophagus, intestine, oviduct, and ureter. While peristaltic pumping of a Newtonian fluid is well understood, in many important settings, as in the fluid dynamics of reproduction, the fluids have non-Newtonian responses. Here, we present a numerical method for simulating an Oldroyd-B fluid coupled to contractile, moving walls. A marker and cell grid-based projection method is used for the fluid equations and an immersed boundary method is used for coupling to a Lagrangian representation of the deforming walls. We examine numerically the peristaltic transport of a highly viscous Oldroyd-B fluid over a range of Weissenberg numbers and peristalsis wavelengths and amplitudes. |
|
Abstract: Motivated by the intriguing motility of spirochetes of helically shaped bacteria that screw through viscous fluids due to the action of internal periplasmic flagella, we examine the fundamental fluid dynamics of superhelices translating and rotating in a Stokes fluid. A superhelical structure may be thought of as a helix whose axial centerline is not straight, but also a helix. We examine the particular case in which these two superimposed helices have different handedness, and employ a combination of experimental, analytic, and computational methods to determine the rotational velocity of superhelical bodies being towed through a very viscous fluid. We find that the direction and rate of the rotation of the body is a result of competition between the two superimposed helices; for small axial helix amplitude, the body dynamics is controlled by the short-pitched helix, while there is a crossover at larger amplitude to control by the axial helix.We find far better, and excellent, agreement of our experimental results with numerical computations based upon the method of Regularized Stokeslets than upon the predictions of classical resistive force theory. |
|
Abstract: The coordinated beating of motile cilia is responsible for ovum transport in the oviduct, transport of mucus in the respiratory tract, and is the basis of motility in many single-celled organisms. The beating of a single motile cilium is achieved by the ATPdriven activation cycles of thousands of dynein molecular motors that cause neighboring microtubule doublets within the ciliary axoneme to slide relative to each other. The precise nature of the spatial and temporal coordination of these individual motors is still not completely understood. The emergent geometry and dynamics of ciliary beating is a consequence of the coupling of these internal force-generating motors, the passive elastic properties of the axonemal structure, and the external viscous, incompressible fluid. Here, we extend our integrative model of a single cilium that couples internal force generation with the surrounding fluid to the investigation of multiciliary interaction. This computational model allows us to predict the geometry of beating, along with the detailed description of the time-dependent flow field both near and away from the cilia. We show that synchrony and metachrony can, indeed, arise from hydrodynamic coupling. We also investigate the effects of viscosity and neighboring cilia on ciliary beat frequency. Moreover, since we have precise flow information, we also measure the dependence of the total flow pumped per cilium per beat upon parameters such as viscosity and ciliary spacing. |
|
Abstract: Mammalian fertilization requires the coordinated activity of motile spermatozoa, muscular contractions of the uterus and oviduct, as well as ciliary beating. These elastic structures generate forces that drive fluid motion, but their configurations are, in turn, determined by the fluid dynamics.We review the basic fluid mechanical aspects of reproduction, including flagellar/ciliary beating and peristalsis.We report on recent biological studies that have shed light on the relative importance of the mechanical ingredients of reproduction. In particular, we examine sperm motility in the reproductive tract, ovum pickup and transport in the oviduct, as well as sperm-egg interactions.We review recent advances in understanding the internal mechanics of cilia and flagella, flagellar surface interaction, sperm motility in complex fluids, and the role of fluid dynamics in embryo transfer.We outline promising computational fluid dynamics frameworks that may be used to investigate these complex, fluid-structure interactions. |
|
Abstract: We have developed a fluid-mechanical model of a eucaryotic axoneme that couples the internal force generation of dynein molecular motors, the passive elastic mechanics of microtubules, and forces due to nexin links with a surrounding incompressible fluid. This model has been used to examine both ciliary beating and flagellar motility. In this article,we showpreliminary simulation results for sperm motility in both viscous and viscoelastic fluids, as well as multiciliary interaction with a mucus layer. |
|
Abstract: The method of regularized Stokeslets is a Lagrangian method for computing Stokes flow driven by forces distributed at material points in a fluid. It is based on the superposition of exact solutions of the Stokes equations when forces are given by a cutoff function. We present this method in three dimensions, along with an analysis of its accuracy and performance on the model problems of flow past a sphere and the steady state rotation of rigid helical tubes. Predicted swimming speeds for various helical geometries are compared with experimental data for motile spirochetes. In addition, the regularized Stokeslet method is readily implemented in conjunction with an immersed boundary representation of an elastic helix that incorporates passive elastic properties as well as mechanisms of internal force generation. |